Posted October 12, 2012 in Blog, Ulthera, Uncategorized
News ReleaseUlthera Receives First-Ever FDA Clearance for
Non-invasive Lift of Skin on the Neck and Under the Chin
Non-surgical Ultherapy ultrasound procedure now used to tighten turkey necks and smooth out double-chins
Mesa, Ariz. – Oct. 10, 2012 – Ulthera, Inc. – a global, high-growth medical device company pioneering aesthetic and medical applications using its therapeutic ultrasound platform technology – today announced that its ultrasound platform device, the Ulthera System, has received FDA clearance to non-invasively lift lax tissue on the neck and the submentum (under the chin).
This is the second lift indication that the System has received; the first cosmetic lift indication was granted in 2009, when the Ulthera System was cleared as a facial treatment with a specific indication for non-invasive eyebrow lift. To date, the Ulthera System remains the only energy-based device with an indication for lift.
The Ulthera System – which combines time-honored ultrasound imaging with focused ultrasound therapy – is used in Ultherapy, a face and neck treatment with well over 100,000 patient procedures performed worldwide.
“This latest validation of the Ulthera System and Ultherapy procedure is testament to the results our clinicians report globally,” said Matthew Likens, president and CEO of Ulthera. “We know from our customers and their patients that sagging skin under the chin and on the neck is a significant issue. Patient demand for a non-invasive lift on the face and neck with just one treatment and no downtime is well established.”
“As an FDA-cleared facial treatment for more than three years, many clinicians have noted results outside the indicated brow area,” said principal investigator Jeffrey Kenkel, M.D., Professor and Vice-Chairman of Plastic Surgery at UT Southwestern Medical Center. “The ability to specifically claim lifting in another area, however, required solid quantitative data that would support noticeable and measureable results in these areas.”
“This clearance for the neck and under the chin is just one in a series of many new clinical indications we are pursuing in the years ahead,” Likens continued. “Utilizing our tried-and-true ultrasound technology, our customers can expect to see continued investment by Ulthera to expand our platform technology’s treatment capabilities.”
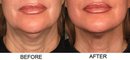
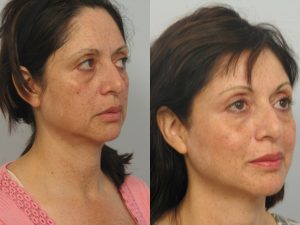
Photos “Before and After” the procedure are available for viewing: http://www.ultherapy.com/Ultherapy-Before-And-After-Photos
About Ulthera
Ulthera®, Inc. is a global, high-growth medical device company pioneering aesthetic and medical applications using its therapeutic ultrasound platform technology. The Ulthera® System is the first and only energy-based device to receive FDA clearance for a non-invasive aesthetic lift indication. It is used in a face and neck procedure known as Ultherapy®, which is cleared to lift skin on the brow, neck and under the chin. Founded in 2004 and based in Mesa, Ariz., Ulthera is a privately held company backed by top-tier venture capital firms, New Enterprise Associates and Apposite Capital. For more information, visit www.ultherapy.com.
Dr. Michael Persky and Dr. Sarmela Sunder are located in Encino, California and Beverly Hills, California (The Lasky Clinic) but service all of Los Angeles and the San Fernando Valley, including, Beverly Hills, Hollywood, Hancock Park, Brentwood, Santa Monica, Pacific Palisades, Malibu, Sherman Oaks, Studio City, Calabasas, Woodland Hills, Tarzana, Westlake, Thousand Oaks, Agoura Hills and more. Please subscribe to our blog by clicking the link above, right. Thank you!




